When you’re dealing with Dangerous Goods, there are many physical and chemical properties that you must consider if you’re to ensure the safe handling and storage of these substances. One of the most commonly-used chemicals, found in workplaces across Australia and the globe, are chemicals known as flammable substances. These Dangerous Goods are recognised for their ability to ignite and burn when they are brought into contact with an ignition source. Because of the flammable nature of these hazardous substances, extreme caution must be taken if your business is carrying this type of chemical. In this blog, we’re explaining everything you need to know about flammable substances, including their flash point, auto-ignition temperature and flammability limits. We’ll also look at the various classes of flammable substances and explain their unique chemical properties, so you can better understand the risks associated with their use.
Defining Flammable Substances
Flammable substances are defined as substances that will ignite and continue to burn when brought into contact with an ignition source. Flammable substances can exist in a solid, liquid or gaseous state.
Most flammable liquids are highly volatile chemicals which emit hazardous vapours. When these vapours mix with air, they form a flammable mixture that easily ignites in the presence of an ignition source — such as a naked flame, pilot light, hot surface or power point.
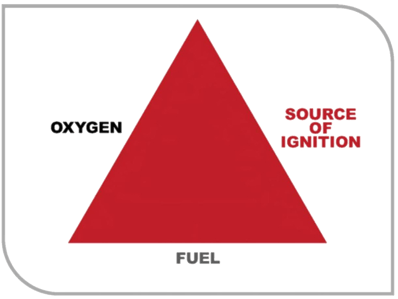 Flammable substances are defined by their ability to ignite and burn in the presence of an ignition source.
Flammable substances are defined by their ability to ignite and burn in the presence of an ignition source.
Flammable Gases, Liquids and Solids
The Australian Dangerous Goods (ADG) Code classifies flammable gases, liquids and solids into 3 different classes of Dangerous Goods.
The Dangerous Goods classes of flammable substances include:
- Class 2.1 Flammable Gases
- Class 3 Flammable Liquids
- Class 4 Flammable Solids
 Flammable gases, flammable liquids and flammable solids are all classed as flammable substances.
Flammable gases, flammable liquids and flammable solids are all classed as flammable substances.
The ADG Code also provides definitions for each of these classes — as well as their chemical subdivisions.
However, it’s important to understand the factors involved with determining the flammability of a substance. When defining a flammable substance as a gas, liquid or solid, there are a range of factors which must be considered.
To understand the flammability of a chemical, you must consider its:
- Flash point
- Auto-ignition temperature
- Flammability limits
What Is Flash Point?
Flammable liquids are highly volatile chemicals. This means that they evaporate and give of flammable vapours at low temperatures. This is due to the forces of attraction between flammable liquid molecules; as they are relatively weak, very little heat energy is required to break these forces and cause the molecules to escape as gases.
The flash point of a liquid is the temperature at which a flammable liquid will give off enough flammable vapours to ignite in the presence of an ignition source. This point will depend on the volatility of the substance. Petrol, which is very volatile, has a flashpoint of -43 °C, while the kerosene, which is less volatile, has a flashpoint between 37 - 65 °C.
 The flash point of a flammable liquid is the lowest temperature at which the liquid will emit enough flammable vapours to ignite.
The flash point of a flammable liquid is the lowest temperature at which the liquid will emit enough flammable vapours to ignite.
What Is The Auto-Ignition Temperature?
Any combustible substance will burn if it’s subjected to enough heat. The lowest temperature at which a flammable or combustible liquid will ignite — and continue to combust without the presence of a spark or flame — is defined as the auto-ignition temperature.
The difference between flash point and auto-ignition temperature, is that the flash point is the point in which a flammable liquid will ignite with an unlimited temperature. On the other hand, auto-ignition temperature is defined as the lowest temperature at which a substance will ignite and continue to combust.
To determine the flash point of a flammable liquid, the chemical is often exposed to a spark. A spark can have a temperature anywhere between 1000 - 1600 °C.
Generally, the auto-ignition temperature of a hydrocarbon will decrease with an increase in molecular mass. This is different to the flashpoint of a substance which decreases with decrease in molecular mass.
How Do You Define Flammability Limits?
For Class 3 Flammable Liquids to ignite and continue to burn, there must be a certain concentration of flammable vapours present in the air.
These vapour levels are referred to as the lower flammability limit (LFL) and the upper flammability limit (UFL).
Understanding Lower Flammability Limit
The lower flammability limit is the lowest concentration of flammable vapours that are required for the mixture to ignite in the presence of an ignition source.
Concentrations of flammable vapours which fall below the lower flammability limit will not be sufficient enough for the chemical to ignite.
Explaining Upper Flammability Limit
The upper flammability limit is the highest concentration of flammable vapours that will ignite in the presence of an ignition source.
If the concentration of flammable vapours exceeds the UFL, there will not be enough oxygen present for the mixture to ignite and continue to combust.
 To determine the flammability of a chemical, you must consider its flash point, auto-ignition temperature and flammability limits.
To determine the flammability of a chemical, you must consider its flash point, auto-ignition temperature and flammability limits.
What Are The Different DG Classes Of Flammable Substances?
We’ll now go into further detail about the different Dangerous Goods classes of flammable substances, so you can understand the volatility (and potential risks) of each different chemical class.
Class 2.1 - Flammable Gases
The ADG Code defines flammable gases as:
Gases which at 20 °C and a standard pressure of 101.3 kPa:
(i) are ignitable when in a mixture of 13 per cent or less by volume with air; or
(ii) have a flammable range with air of at least 12 percentage points regardless of the lower flammable limit. Flammability should be determined by tests or by calculation in accordance with methods adopted by ISO (see ISO 10156: 1996). Where insufficient data are available to use these methods, tests by a comparable method recognized by the competent authority may be used;
Some examples of flammable gases include:
- Acetylene
- Liquefied Petroleum Gas
- Hydrogen
- Methane
- Propane
Class 3 - Flammable Liquids
The ADG Code defines flammable liquids as:
Flammable liquids are liquids, or mixtures of liquids, or liquids containing solids in solution or suspension (for example, paints, varnishes, lacquers, etc., but not including substances otherwise classified on account of their dangerous characteristics) which give off a flammable vapour at temperatures of not more than 60 °C, closed-cup test, or not more than 65.6 °C, open-cup test, normally referred to as the flash point. This class also includes:
(a) liquids offered for transport at temperatures at or above their flash point; and
(b) substances that are transported or offered for transport at elevated temperatures in a liquid state and which give off a flammable vapour at a temperature at or below the maximum transport temperature.
Some examples of flammable liquids include:
- Petrol
- Diesel
- Kerosene
- Acetone
- Benzene
- Methylated Spirits
- Toluene
- Diethyl Ether
IMPORTANT: When storing Class 3 Flammable Liquids you must ensure that you are reducing the risk of fire, explosion, asphyxiation and human harm. Only chemical cabinets and stores that have been manufactured to meet the requirements of Australian Standard AS 1940:2017 will provide adequate protection from chemical hazards.
Class 4 - Flammable Solids
The ADG Code divides Class 4 Flammable Solids into 3 subdivisions. Let’s have a look at each of these subdivisions in detail below:
Class 4.1 - Flammable Solids
Solids which, under conditions encountered in transport, are readily combustible or may cause or contribute to fire through friction; self-reactive substances which are liable to undergo a strongly exothermic reaction; solid desensitized explosives which may explode if not diluted sufficiently.
Some examples of flammable solids include:
- Processed metals
- Metallic sodium
- Potassium
Class 4.2 - Spontaneous Combustibles
Substances which are liable to spontaneous heating under normal conditions encountered in transport, or to heating up in contact with air, and being then liable to catch fire.
Some examples of Class 4.2 Dangerous Goods include:
- Copra
- Phosphorous
Class 4.3 - Dangerous When Wet
Substances which, by interaction with water, are liable to become spontaneously flammable or to give off flammable gases in dangerous quantities.
Some examples of Class 4.3 Dangerous Goods include:
- Metal Powders
- Alkali Metals
- Activated Carbon
- Aluminium Phosphide
- Sodium Batteries
Class 4.3 Dangerous Goods will ignite or emit flammable gases if they interact with water.
Is Your Business Carrying Any Type Of Flammable Substance?
As we’ve discussed in this blog, flammable substances come in many different forms. Whether it be solids, liquids or gases, all flammable substances pose risks upon the people, property and the environment of your organisation. To reduce the risks associated with the handling and storage of flammable substances, it’s vital that staff, supervisors and contractors are familiar with their dangerous chemical properties. By having a good understanding of the flammable substances that your business is using, you’ll be able to implement risk control measures to reduce the potential for fire, flashback, explosion, human harm and environmental damage. Would you like more information on how to reduce the risk of flammable liquids? You can quickly access our free eBook by clicking on the below image. Download it and read it today to learn more about reducing the risks associated with flammable liquids.
Joining the team as a Dangerous Goods Storage Consultant, Melissa Hampton became Storemasta's Marketing Manager in late 2021. With extensive knowledge and experience in chemical compliance, Melissa is responsible for leading the Marketing team and helping shape their marketing strategy. In her spare time, you can find Melissa hiking, swimming and enjoying the great outdoors in beautiful north-west Tasmania.
