Corrosive substances can pose a serious risk to the workplace. As a destructive substance, it's important to understand the hazardous properties of corrosive substances, so you can ensure they’re used and stored in a safe, compliant manner.
What are Corrosive Substances?
Corrosive substances are defined as materials that can attack and destroy, by chemical action, living tissue, organic compounds and metal. Recognised as Class 8 Corrosive Substances, they have the ability to cause severe damage to people, property and the environment.
Why are Corrosive Substances Harmful?
Corrosive substances are very dangerous because they destroy living cells and tissue — from the first moment of contact. Cell and tissue destruction is visible, irreversible and very, very painful. It’s important to remember that severe exposure to corrosive substances can be fatal.
Corrosive chemicals can cause human harm through exposure if they’re inhaled, swallowed or splashed into the eyes or onto the skin of workers. Corrosive substances can also quickly damage or destroy property, transport and other goods that they come into contact with, such as metal or stone. Class 8 liquids may seep or spill into the natural environment through, causing harm to animals, wildlife and the ecosystem who have contact with contaminated water or soil.
However, as with any type of hazardous chemical or dangerous goods, the individual chemical and physical properties of the substance will determine how harmful the chemical really is. When we talk about corrosives, it’s the pH level of the substance that is a key measure of its corrosive properties.
![]()
Corrosives can harm people, property and the environment - including the destruction of storage containers and facilities.
What is a pH Level?
Ranked on a scale between 1 and 14, pH is a measurement of how acidic or basic a water-soluble substance is.
If a substance is considered neutral — such as pure water — it will have a pH level of 7. As the substance becomes increasingly acidic, the pH level decreases from 7.
Anything with a pH level above 7 indicates that the substance is alkaline. As the pH value increases, so does the alkaline level of the substance.
For example, lemon juice has a pH level of 2, coffee may have a pH level of 5 and rainwater has a value of 5.5. While hand soap may have a pH level of 10 and bleach may have a pH level of 12.
![]() pH Scale
pH Scale
It’s important to note that a pH of 4 is 10 times more acidic that a pH of 5 — and 100 times more acidic than a pH of 6. Likewise, a pH of 13 is 10 times more alkaline than a pH of 12 — and 100 times more alkaline than a pH of 11.
Are Acids Corrosive?
Class 8 Corrosive Substances are divided into two categories: acids and bases. Acids have a pH level that’s lower than 7, while bases have a pH level that’s greater than 7. The more acidic or more alkaline a substance is, the more effective it will be as a corrosive substance.
As acids are classed as corrosive chemicals, great care must be taken to ensure that they don’t come into contact with people, property or the environment. However, just how corrosive an acid is will determine the exact procedures for its storage and handling.
The lower the acid’s pH level is, the more corrosive the acid will be. For example, a strong acid may have a pH level of 2, while a weak acid may have a pH level closer to neutral, such as 6.
![]()
Corrosion can occur when Class 8 chemicals spill or emit corrosive vapours.
The same principle applies when you’re determining how corrosive a base will be. The higher the pH level is on the scale, the more corrosive the Class 8 base will be. A weak base may have a pH level of 9 while a strong base may have a pH level of 11.
Examples Of Corrosive Acids
What are some examples of commonly used acids and bases? We’ll now discuss some examples of commonly used acids and bases that you may find in your own workplace.
Hydrochloric Acid
Hydrochloric acid (HCL) has a pH level of 1.1 at 38% concentration. If this acid accidently came into contact with your eyes or skin, it would immediately begin to dissolve your skin tissue. HCL is commonly used in the production of batteries, fireworks, leather and building materials. It is also commonly used in water and salt purification. Your body also produces Hydrochloric acid to assist your digestive system.
Sulfuric Acid
The sulfuric acid pH level is 0.5 at a concentration of 33.5% — which is the equivalent to the concentration of sulfuric acid used in lead-acid batteries. Sulfuric acid (H2So4) is one of the most important industrial chemicals. It plays a part in the production of a lot of manufactured goods. Despite being primarily used in the production of fertilizers such as superphosphates, sulfates and ammonium phosphates, sulfuric acid is also widely used in the production of dyes, paints, fabric pigments, explosives, lubricants, metals and batteries. Sulfuric acid is a dehydrating agent and reacts violently with water. When in contact with water, sulfuric acid will boil and splash. If sulfuric acid is released into the atmosphere, it can cause acid rain which is very harmful to the environment.
![]()
Sulfic acid is a Class 8 Corrosive with a pH level of 0.5.
Nitric Acid
Nitric acid (HNO3) has a pH level of 1.2 at standard commercial concentration of 68%. The uses of Nitric acid are very similar to those of sulfuric acid. Nitric acid is used in the production of ammonium nitrate, plastics, dyes and explosives such as nitroglycerin and TNT. When nitric acid is combined with HCL, a fuming liquid, known as aqua regia is formed capable of dissolving gold and platinum. Nitric acid is also utilised in the medical industry to remove warts and as a colorimetric test to distinguish drugs. When exposed to the skin, nitric acid can cause severe burns, ulceration, dermatitis and yellow staining. As a corrosive substance, nitric acid is capable of dissolving many materials including most metals. For ultimate safety nitric needs to be stored in a compliant safety cabinet to prevent the risk of acid burns and damage to expensive property, plant and equipment.
Concerned about corrosive spills?
Access our free spill bunding eBook
Chromic Acid
Chromic acid (H2CrO4) has a pH level of approximately 3.03. It is used as a cleaning agent and in the manufacturing of glazes, colored glass and chromium plating. In the past, it has commonly been used as a bleach to dye hair. Due to the high impact chromic acids have on the environment and human health, it’s no longer used for this purpose. Again, chromic acid will burn your skin and safe handling and storage is crucial.
Acetic Acid
Acetic acid (CH3COOH) or as we more commonly know it, vinegar, has a pH of 2.4 at 5% dilute solution. Acetic acid is primarily used in the production of vinyl acetate monomer, followed by ester production and used to create solvents for inks, paints and coatings. Acetic acid has proved to be widely useful in the medical industry, especially in the treatment of cancer amongst many other conditions such as ear infections. And of course, acetic acid is used in food production. Surprisingly enough, vinegar is only 4-18% acetic acid by mass. Despite seeming reasonably harmless, acetic acid, specifically in concentrated amounts can be considerably harmful to human health and the environment.
Some Examples Of Corrosive Bases
There are many types of corrosive bases that are commonly used in workplaces across a wide range of industries including manufacturing, mining, printing and the production of pharmaceuticals. Some examples of Class 8 bases include:
Ammonium Hydroxide
Ammonium hydroxide (NH4OH) has a pH of 10.09 and is often used as a glass cleaning agent in various commercial and industrial products. You will also find ammonium hydroxide in food items where it is used as a food additive to the correct level of acidity. This corrosive base is also commonly used as a refrigerant and can be found in a range of products including detergents, textiles, soaps, ceramics, pharmaceuticals, inks and explosives. As ammonium hydroxide may cause extensive tissue damage if it is exposed to the skin, it must be stored in a safe and compliant manner.
Potassium Hydroxide
Potassium hydroxide (KOH) has a pH of 10.98 and is commonly used in the production of fertilisers, biodiesels and soft soaps. Potassium hydroxide is used as an electrolyte in a process commonly referred to as chemical cremation or resomation.
Sodium Hydroxide
Sodium Hydroxide (NaOH) has a pH of 13. Although it is predominantly used in the paper industry, it plays an important role in tissue digestion and in the manufacturing of sodium salts and detergents. Sodium hydroxide can also be found in cleaning agents and as a pH regulator in organic synthesis and metal production. If sodium hydroxide is not handled and stored in a safe corrosive storage cabinet, it can be dangerous to human health and the environment.
Sodium Hypochlorite
Sodium hypochlorite (NaClO) has a pH of approximately 13 at 10-15% concentration. It is used in various industries, including waste management, as a bleach or disinfectant. Sodium hypochlorite also has other applications, including as an antiseptic in the medical industry and as a pesticide in the agricultural industry.
Storage Considerations
Now that you have a better understanding of corrosive substances and their pH levels, it’s important to consider proven strategies that will minimise their harm. This includes the safe storage of corrosive substances.
Regardless of whether you’re storing acids or bases at your site, you must refer to Section 9 of every safety data sheet (SDS) to determine the physical and chemical properties of each substance — including the pH level.
Those acids and bases that are regarded as highly corrosive — that is producing sufficient corrosive gases to corrode metal — must be stored in a compliant polyethylene cabinet. This type of storage cabinet won’t be susceptible to corrosion with highly corrosive acids and bases.
Weaker acids and bases may be stored in a Class 8 metal cabinet that’s equipped with drip trays and sump lining to prevent the corrosion of the sheet steel construction.
Always ensure that acids and bases are stored separately as they are regarded as incompatible substances. This can be the storage of acids and bases in separate Class 8 cabinets or stores, or within the same indoor cabinet that has the provision of a compliant segregated compartment.
Understanding Corrosive Acids & Bases
So, with the serious risks associated with Class 8 chemicals, how can you properly manage the handling and storage of corrosive substances in your workplace?
First, it’s important to understand the properties of the substance so you can formulate the right strategy to ensure that it’s safely used and contained. And one of the key measures of a liquid’s corrosive properties is its pH level. We always suggest conducting an onsite risk assessment to properly determine the risks associated with your worksite. When assessing chemical risks in the workplace, you must ensure that the safety data sheets for each substance are available, so that the hazards are accurately identified and incompatible substances are sufficiently isolated.
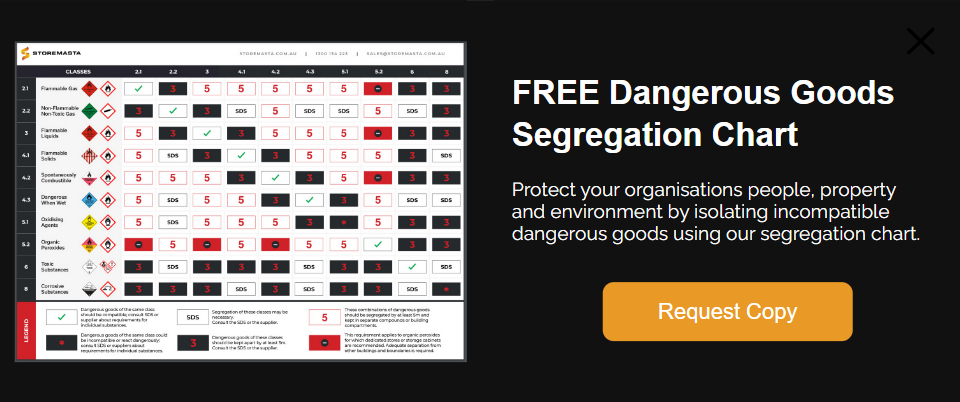
To learn more about the safe storage of Class 8 Corrosive Substances, why not download our free dangerous goods segregation chart. This handy tool will help you separate incompatible substances in the workplace, so your staff, contractors and customers can remain protected. Get your copy today to find out more.
Joining the team as a Dangerous Goods Storage Consultant, Melissa Hampton became Storemasta's Marketing Manager in late 2021. With extensive knowledge and experience in chemical compliance, Melissa is responsible for leading the Marketing team and helping shape their marketing strategy. In her spare time, you can find Melissa hiking, swimming and enjoying the great outdoors in beautiful north-west Tasmania.